Chemistry only
Making fertilisers in the lab
Ammonium sulfate and ammonium nitrate are two common compounds that are used as
fertilisers. They are both easy
to make in the lab by carrying out a simple neutralisation reaction. Word and symbolic equations for the neutralisation reaction that can be used to prepare ammonium sulfate are shown below:
ammonium hydroxide(aq) + sulfuric acid(aq) → ammonium sulfate(aq) + water(l)
2NH4OH(aq) + H2SO4(aq) → (NH4)2SO4(aq) + 2H2O(l)
The diagram below outlines a method that can be used to make ammonium sulfate crystals by carrying out a simple neutralisation
reaction:
Making ammonium sulfate by neutralisation using ammonium hydroxide solution
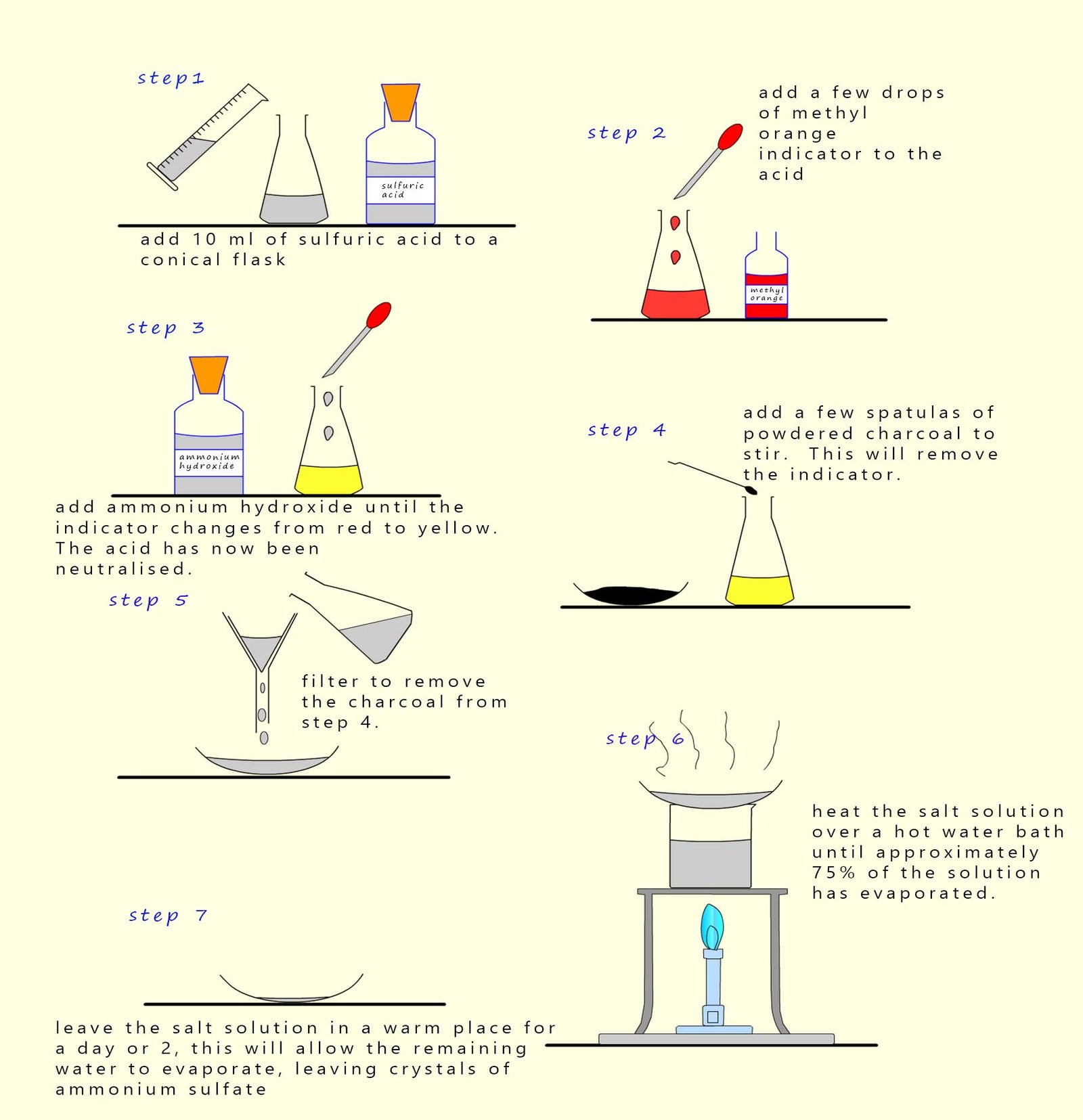
Instructions for making ammonium sulfate crystals
- Step 1. - To make a salt called a sulfate you must use sulfuric acid.

- Step 2.- A few drops of methyl orange indicator are added to the sulfuric acid. Methyl orange indicator is
red in acid and
yellow in neutral/alkaline solutions.
- Step 3.-To neutralise the sulfuric acid slowly add;
with constant stirring the alkaline ammonium hydroxide solution. The
sulfuric acid
will have been neutralised when the indicator jus
turns yellow.
- Step 4. - The indicator; in this case methyl orange;
is a coloured dye and must be removed otherwise the final crystals of
ammonium sulfate will
be covered in the coloured indicator. Now charcoal is good at
removing smells, stains and odours. So simply add a few
spatulas full of powdered charcoal and stir for a few minutes to remove the methyl orange indicator from the solution.
- Step 5 - The charcoal needs to be removed from the solution now. This is easily done by simple
filtration.
- Step 6 - Heat the salt solution over a
water bath until approximately 75% of the solution has evaporated.
- Step 7 - Place the remaining salt solution on an evaporating basin and leave in a warm sunny spot to allow the remaining
water to evaporate and the ammonium sulfate crystals to grow.
You can alter the method above to make ammonium nitrate by simply using nitric acid instead of sulfuric acid in step 1.
Next



